READDI helps build a Therapeutics Coalition
Global leaders from government, the private sector and academia met in London to explore a coordinated approach to developing antiviral therapeutics, a pandemic response cornerstone.

By READDI, July 9, 2024 — When it comes to research and development for pandemic therapeutics, there’s good news and bad news, said READDI co-founder Dr. Nat Moorman at a June 24 London workshop, “Towards building a Therapeutics Coalition.” He was speaking to a roomful of fellow participants — international leaders from government, the private sector and academia — who gathered to explore the practical steps towards a more coordinated approach to discovering therapeutics for high-risk virus families.
The bad news? The R&D pipeline is mostly bare, Moorman said.
Only SARS-CoV-2, Ebola and Lassa — three viruses from three different virus families — have any candidates in Phase 2 or 3 trials. That leaves seven other priority virus families with few to no clinical candidates. “We have a lot of work to do and a lot of gaps to fill,” said Moorman, associate professor in the UNC School of Medicine’s microbiology and immunology department.
The good news? Five years ago, there was no R&D funding for SARS-CoV-2. But after the virus emerged, governments, philanthropy and industry accelerated the discovery and development of life-saving therapeutics for COVID-19.
With the right funding and focus, Moorman said, the same “can be done for any of the viruses on the priority list. It can be done for any virus family.”
The challenge is finding the funding and focus now, in advance of the next highly contagious viral threat. That’s where a Therapeutics Coalition can help.
READDI’s role in the Coalition is to collaborate with colleagues in antiviral drug discovery and development. This is READDI’s mission, and it feels great that so many of our peers want to pull in the same direction.”
Hosted by the Wellcome Trust and spearheaded by the International Pandemic Preparedness Secretariat, the coalition workshop was co-organized by READDI, the World Health Organization, Drugs for Neglected Diseases initiative (DNDi), IFPMA, Unitaid, INTREPID Alliance, Medicines Patent Pool and the 100 Days Mission Therapeutics Working Group.
The 100 Days Mission was embraced by the G7 and G20 in 2021 to ensure the global availability of diagnostics, therapeutics and vaccines within the first 100 days of a pandemic threat. Other groups lead the diagnostics and vaccines pillars. The workshop was a major step toward establishing a Therapeutics Coalition to lead the third pillar.
The role of therapeutics
Therapeutics are a cornerstone of an effective pandemic response, especially before vaccines are approved and for populations who are unable to use vaccines. Safe and effective therapeutics reduce mortality and morbidity during pandemics. They ease hospital overcrowding and, when deployed effectively, prevent viral spread.
Therapeutics, however, have been overlooked during past outbreaks. The lack of funding for therapeutics — especially compared to vaccines — significantly hampered efforts to develop and ensure equitable access to COVID-19 treatments. A lack of end-to-end leadership, structure and coordination as exists for vaccines and diagnostics puts therapeutics in a double bind. On the one hand, there is a risk of duplication and wasted resources, while on the other, there is the danger of neglect and a narrow pipeline of drugs.
A Therapeutics Coalition would strengthen the therapeutics value chain from end to end, directing funding and taking collaboration and coordination to the next level. The group’s vision is not for a new entity but a genuine coalition of existing partners, working in alignment with existing global structures such as the WHO R&D Blueprint and the interim medical countermeasures network being established by the WHO, following on from the ACT-Accelerator. Initial focus areas would be discovery/early-stage R&D, clinical trials and regulatory pathways, as well as market shaping and access.
‘Focus on action’
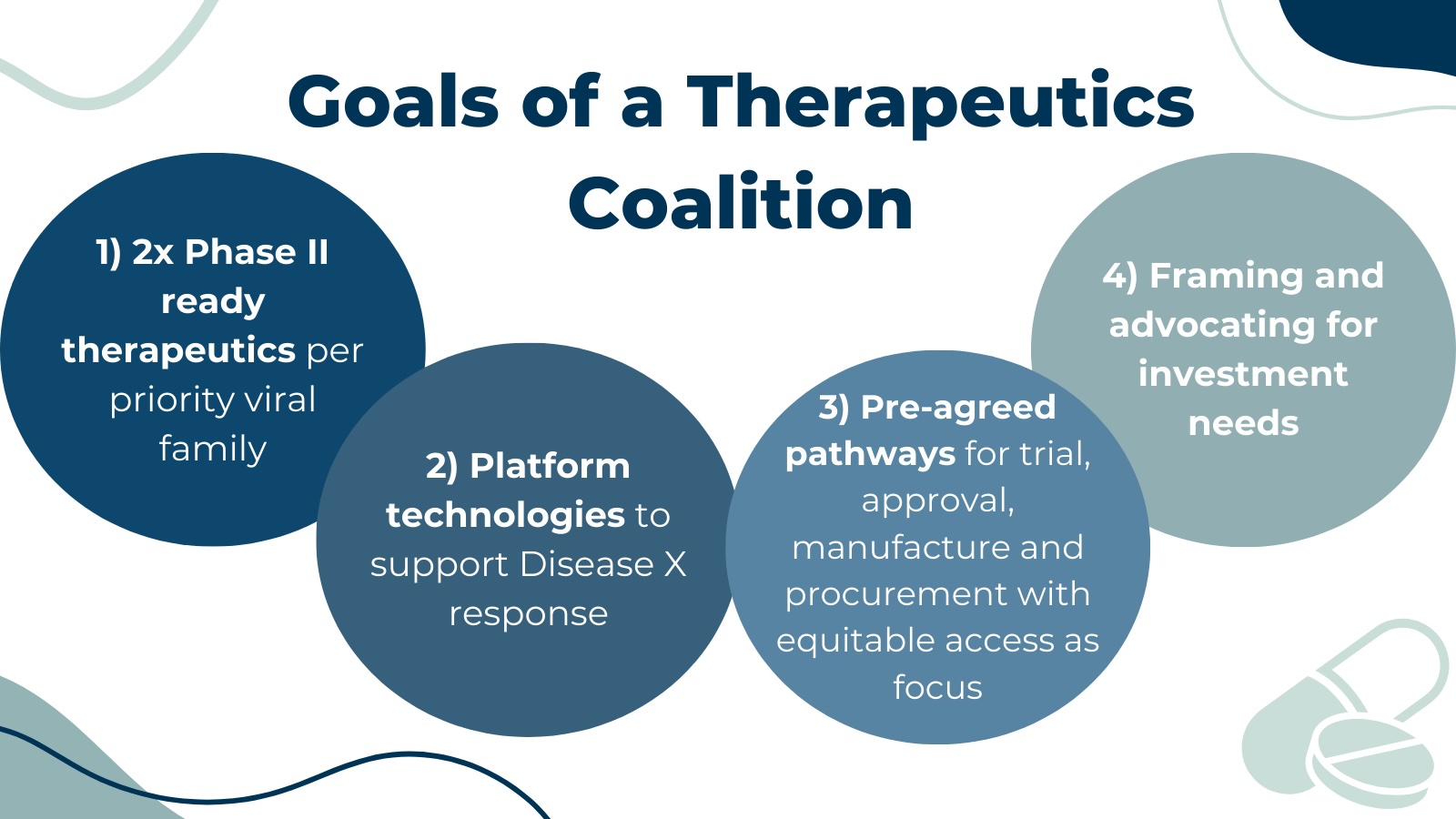
The goals of a Coalition are multifold:
- Ensuring sustained R&D funding throughout the development lifecycle.
- Developing at least two ‘Phase 2 ready’ therapeutic candidates for the viral pathogen families of greatest pandemic potential. Equitable access must be a key principle in development and delivery.
- Developing scientifically rigorous and validated programmable platforms or technologies capable of accelerating the availability of new therapeutics or enhancing existing therapeutics in case of a pandemic.
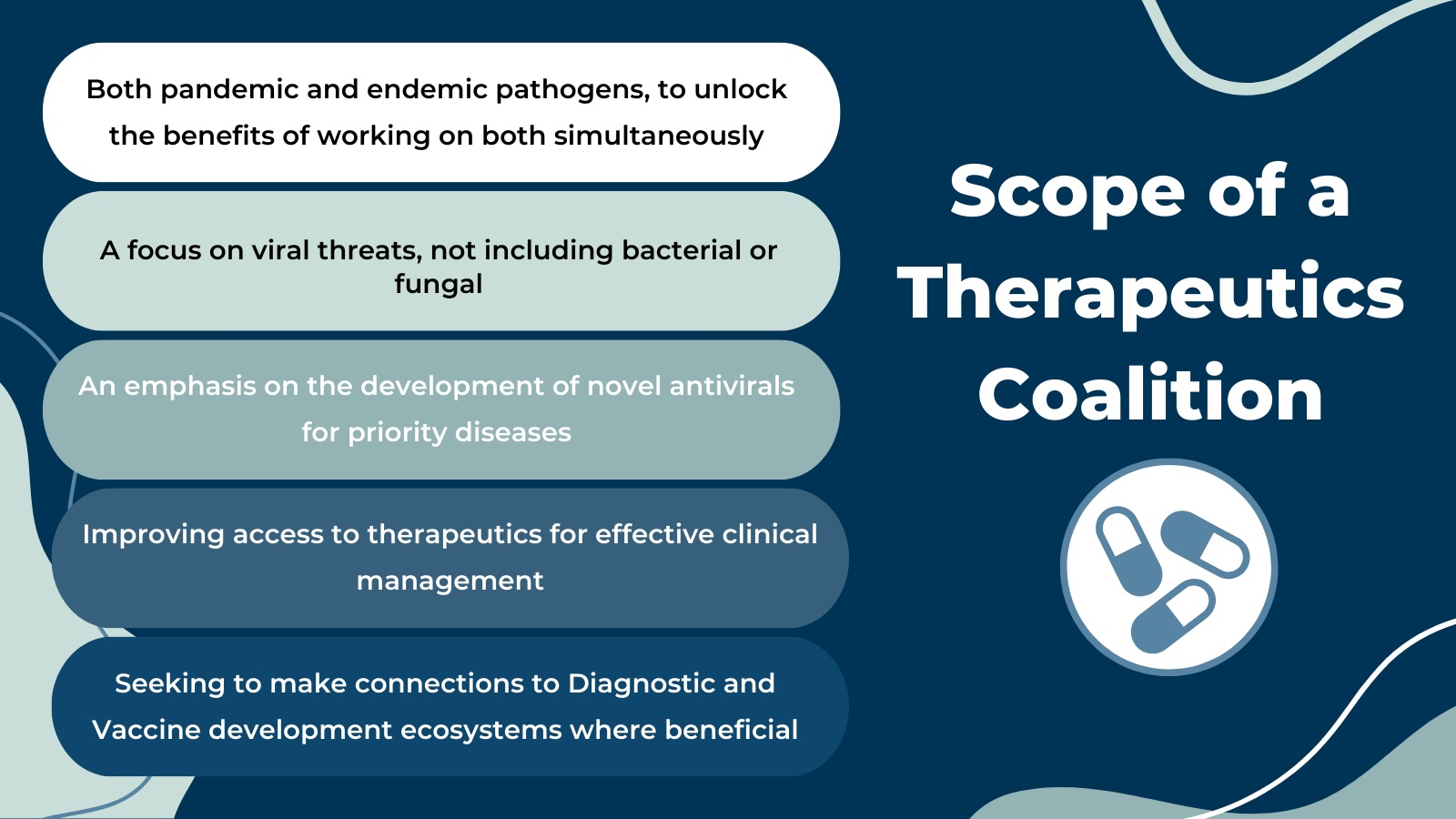
The Coalition effort is focused on both pandemic and endemic viruses to unlock the benefits of working on both simultaneously, including sharing common solutions and increasing investment viability. The emphasis is on novel antivirals for priority diseases and investing to support antiviral development and access, with particular attention to broad-spectrum therapeutics potentially applicable across different viruses of the same family.
The Coalition will target access to therapeutics for adequate clinical management, including smart repositioning of current tools. It seeks to make beneficial connections to diagnostic and vaccine development ecosystems, such as the design of test-to-treat programs.
“The workshop discussions were focused on action. We committed to quickly prioritize next steps that can be accomplished by knowledgeable organizations within the Coalition,” said READDI CEO Jimmy Rosen, who also attended the London workshop. “READDI’s role in the Coalition is to collaborate with colleagues in discovery and development to ensure that a robust antiviral pipeline is comprised of potential therapeutics that will be affordable and accessible to everyone who needs them, where they need them, when they need them. This is READDI’s mission, and it feels great that so many of our peers want to pull in the same direction.”
The June 24 workshop was an early but important step in the creation of a Therapeutics Coalition. Meeting the Coalition’s goals will require the participation of all sectors and all continents.
To find out more and get involved, please contact [email protected].